The number of COVID-19 cases and deaths continues to climb. As a pharmacist, it’s uplifting to see positive news on the horizon — namely the vaccines. The pharmaceutical industry, the U.S. government, scientists and global health agencies have collaborated to develop these vaccines at an unprecedented, yet safe, speed. This was possible because the technology involved in developing these vaccines has been around for years. This, combined with collaboration, led to the development of COVID-19 vaccines.
Here are answers to some of the most common questions people have about the vaccine, as of December 21, 2020.
How were the vaccines developed?
The Pfizer-BioNTech and Moderna are both mRNA vaccines, which stands for messenger ribonucleic acid. This type of vaccine is manufactured in a cell-free environment. For example, you may have heard that some vaccines, like influenza vaccine, require eggs to develop. mRNA technology does not — it is cell free and allows manufacturers to rapidly produce large quantities of vaccine.
How will people get the vaccine?
The vaccine requires two doses and is injected into the shoulder muscle just like the influenza vaccine. The Pfizer vaccine doses are to be given 21 days apart; the Moderna 28 days apart.
Could the vaccine give me COVID-19?
No, this vaccine does not contain the live virus. It cannot cause a COVID-19 infection.
Can the vaccine cause side effects?
Side effects are possible. The most common side effects reported are:
- Pain at the injection site
- Fatigue
- Headache
- Muscle or joint aches
- Chills
- Fever
Most of these side effects were mild to moderate and similar to side effects experienced in other FDA-approved vaccines. Side effects usually happen within 24-48 hours of the injection and are short-lived.
How will the vaccines be distributed?
Both the CDC and the state of Nebraska have comprehensive vaccine plans. These plans guide institutions on how to prioritize initial vaccine supplies. Plans are evolving and updated as new information becomes available.
Here is the Federal plan as of December 22, 2020.
Phase 1A – health care workers and long-term care facility residents.
Phase 1B – persons age 75 and older, and frontline essential workers.
Phase 1C – persons age 64-74 years, persons aged 16-64 with high-risk medical conditions, and other essential workers.
Phase 2 is expected to occur later in spring 2021, and will include the general population.
What age groups will be allowed to receive the vaccine?
The FDA Emergency Use Authorization (EUA) for each vaccine determines the ages of children that can be vaccinated. The Pfizer vaccine may be given to patients 16 and older, and the Moderna vaccine may be given to individuals 18 and older.
Will children get the vaccine?
The Pfizer vaccine was studied in children as young as age 12, but it only has EUA for children 16 and older. The Moderna vaccine was only studied in individuals 18 and older. The FDA determines the ages of children that can be vaccinated.
What are the ingredients in the vaccine?
The ingredients in the two mRNA vaccines with Emergency Use Authorization are:
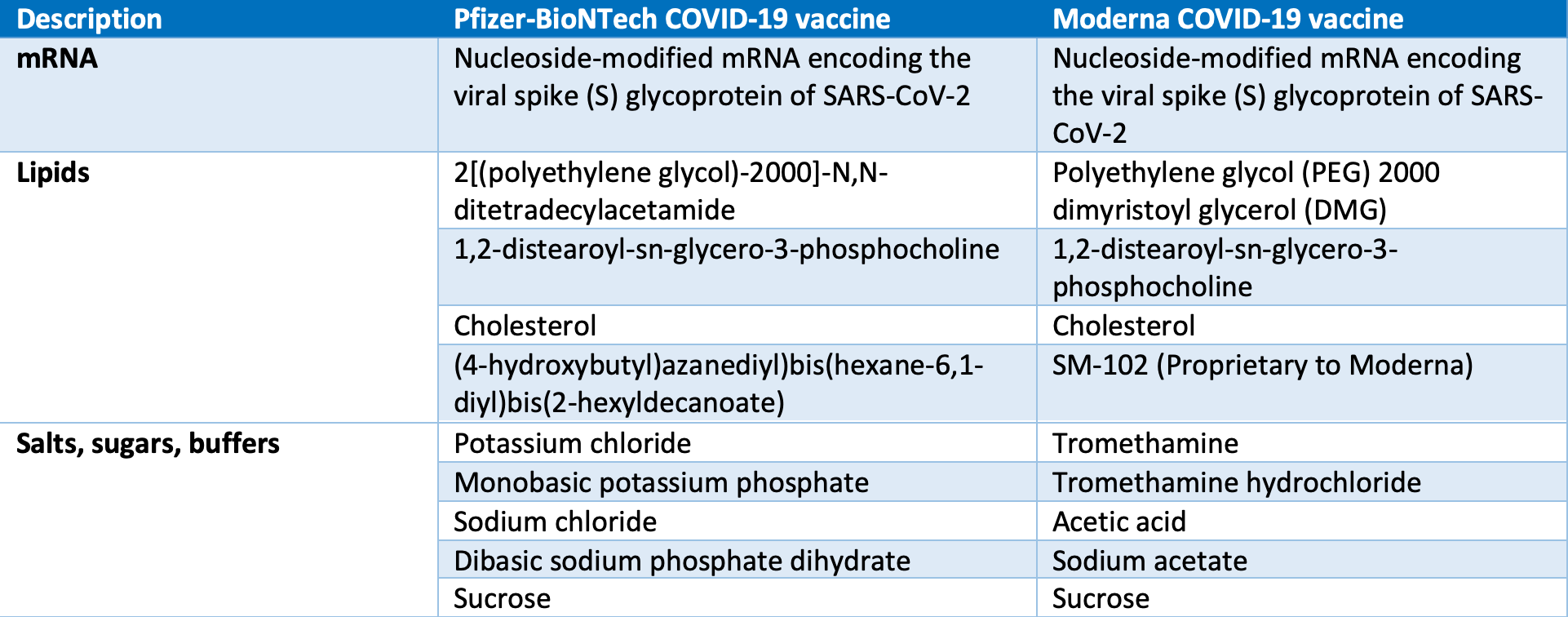
Information via CDC.gov
Do I have to pay for the COVID-19 vaccine?
The government is providing vaccine doses to people across the country at no cost. There may be charges to administer the vaccine.
If I’ve recovered from COVID-19, do I need to be vaccinated? Does immunity after getting COVID-19 last longer than protection from COVID-19 vaccines?
The protection someone gains from having an infection (called natural immunity) varies depending on the disease, and it varies from person to person. Since this virus is new, we don’t know how long natural immunity might last. Early evidence — based on some people — suggests that natural immunity may not last very long, so the vaccine is still needed.
Will the vaccine cause me to have a positive COVID-19 test?
No, but it is possible that a recipient of the vaccine may have positive antibody tests.
Since these vaccines were developed so fast, were any steps in the approval process skipped?
No. mRNA technology is not new — the many years of research allowed the rapid development of the COVID-19 vaccine to occur.
Should pregnant women receive the vaccination? What about those who are breastfeeding?
Pregnant and breastfeeding women were not included in clinical trials, so these risks are not known. Based on current knowledge, scientists believe that mRNA vaccines are unlikely to pose a risk for pregnant women. You should consider your personal risk of contracting COVID-19, the risks of COVID-19 to you and potentially to your fetus, the efficacy of the vaccine, the side effects of the vaccine and the lack of data about the vaccine during pregnancy. The CDC also notes that those who are trying to become pregnant do not need to avoid pregnancy after receiving the Pfizer-BioNTech COVID-19 vaccination.
Can the vaccine impair my fertility?
There is no data showing that the vaccine affects fertility. You may see rumors suggesting this, but it has never been shown. Women who were trying to conceive were excluded from the study. The following statement comes from the CDC: “Those who are trying to become pregnant do not need to avoid pregnancy after Pfizer-BioNTech COVID-19 vaccination.” Please discuss with your health care provider if you have additional concerns.
I’m immunocompromised, should I get the vaccine?
This vaccine does not contain live virus, so it does not pose risk of infectious side effects regardless of immune status. However, the CDC states that persons with immunocompromising conditions might be at increased risk for severe COVID-19. Data is not yet available to establish vaccine safety and efficacy in these groups. You may receive the COVID-19 vaccination if you have no contraindications to vaccination. This is a decision you should make after talking with your health care provider.
How long does immunity last?
It is not yet known how long immunity to COVID-19 lasts, either in a person who recovered from the disease or one who got vaccinated. It is possible that vaccines will require additional booster doses at some point after the first two doses.
What is this idea called herd immunity?
Herd immunity happens when a virus can’t spread because it keeps encountering people who are already protected against infection. Once a large portion of the population is no longer at risk, any new outbreak should halt. Experts estimate that in the U.S., about 70 to 80 percent of the entire population — more than 200 million people — must recover from COVID-19 to stop the pandemic from getting worse. But, that level of infection will lead to large numbers of patients with serious long-term complications and millions of deaths.
This is why the vaccine is so important. Our health care system cannot accommodate that many patients — we are overwhelmed now. When you get the vaccine, you help create herd immunity because the virus can’t spread due to the protection the vaccine provides.
If I get the vaccine, do I still need to wear a mask?
Yes, we all need to continue the same effective practices of wearing a mask, washing our hands and social distancing for the foreseeable future. We are in a long battle with a fierce enemy and we can’t let up, but HOPE is here.
Learn More
Dr. Kevin Reichmuth, pulmonologist with Nebraska Pulmonary Specialties, describes how the vaccine was developed and provides additional information on myths, facts and hope.
Watch Vaccine Development, Facts, Myths & Hope.
Katie Packard, PharmD, Bryan pharmacist, provides information on the vaccine and answers frequently asked questions.
Watch the General Overview and Vaccine Myths & FAQs.

Katie Packard, PharmD
Katie Packard, PharmD, is a clinical pharmacist at Bryan Health.